CALL: 1-855-WE-HEAT-IT
Dew Point Versus Oxygen Content in Vacuum Processing Part 2
Since writing my first article on dew-point versus oxygen analysis in May 2016, we have experienced multiple rounds of brutal heat and humidity that prompted advisories and warnings from Massachusetts to Missouri. We all know that heat waves and droughts are staples of summer weather in America. This past “Summer of 2016” has proven no different. As the cool and dryer autumn and winter air masses moved into Western Pennsylvania, I used the opportunity to compare and contrast all of the environmental factors that are known to affect one of the most important measurements for all heat treaters’, dew point.
Dew Point Considerations in Vacuum Processing
Since the majority of commercial and captive heat treat facilities do not typically operate under controlled environments, the temperature and humidity swings can often be drastic. The environmental changes are especially substantial when comparing the seasonal averages, monthly averages, weekly averages (See Figure 1), or even day light to nighttime daily averages. Temperature, along with adsorption and de-sorption within the gas sampling system also affects the reliability of dew-point readings.
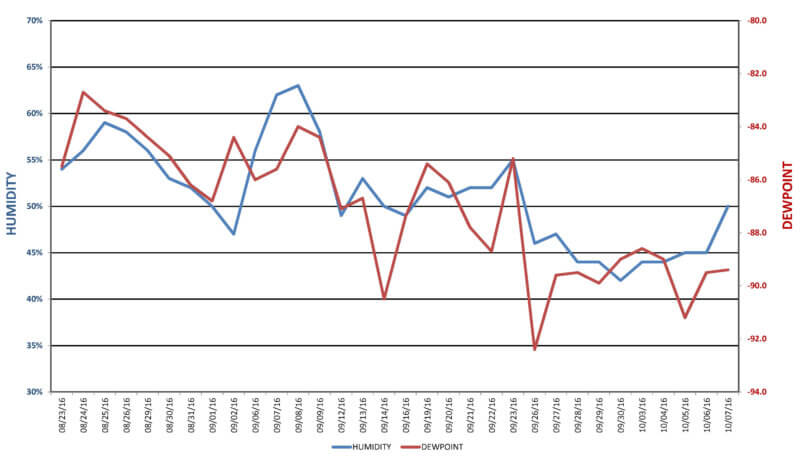
Figure 1 – Humidy-Dewpoint
The precision required of any dew-point analyzer for determining water content within an open atmospheric furnace is much more forgiving. However, when analyzing very dry specialty gases that are used for partial pressures and quenching in vacuum processing, a greater precision is needed. The sensitivity of dew point measurement within very dry ranges is often meaningless. For example, the difference between -110°F and -95°F dew-point is only 2 ppm of water (See Figure 2). Therefore, the accuracy of a dew point instrument within that normal operating range for specialty gases is questionable.
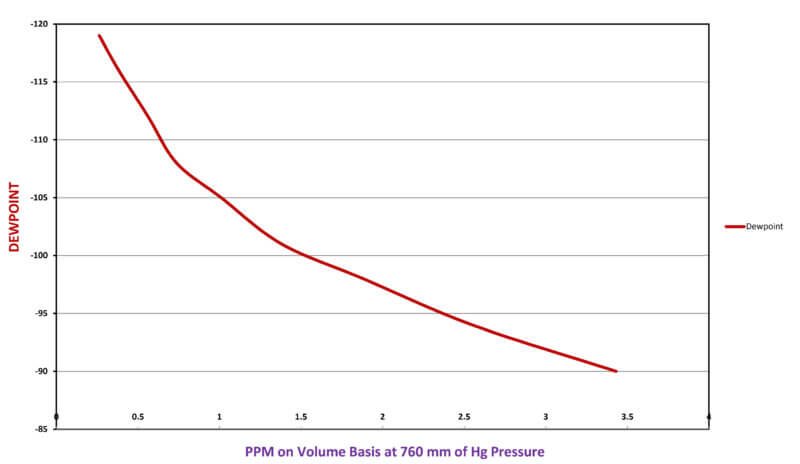
Figure 2 – Dew Point-PPM of Water
Oxygen Analysis Considerations in Vacuum Processing
The present capability to simultaneously compare and contrast dew point readings adjacent to a Trace Oxygen Analyzer on a daily basis has definitely proven the superiority of one measurement versus the other (See Figure 3). Having a consistent signal that is totally independent of temperature and humidity provides a more reliable measurement of the dryness of the gas species that is being analyzed.
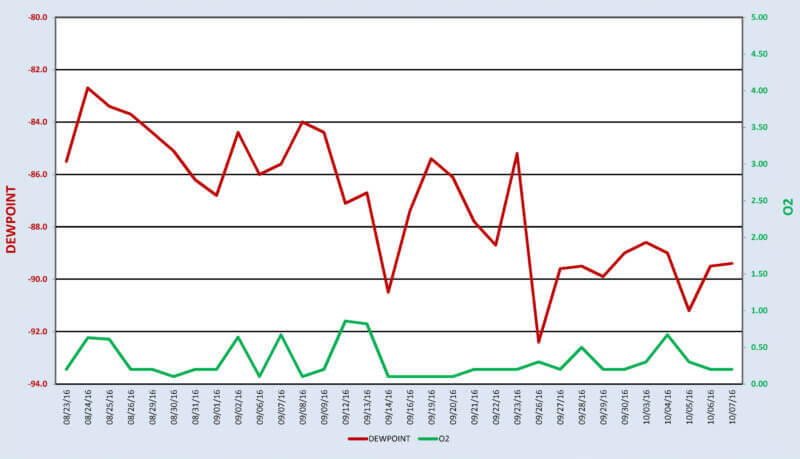
Figure 3 – Dew Point-O2
Calibration
Often a dew point sensor can fail without warning. Typically, an erroneous signal would eventually read extremely dry (e.g. -120° to -150°F). This failure could degrade over a long period of time or overnight. Semiannual calibration of the sensor must be outsourced and maintaining an inventory of a backup instrument can be costly.
Unlike the dew point instruments, the Oxygen Trace Analyzer instrument can be calibrated right on the shop floor by procuring a cylinder of nitrogen gas with exact known oxygen content (See Picture 1).

Picture 1 – Oxygen Trace Analyzer
Calibration of the Oxygen Trace Analyzer instrument using a higher value of Oxygen (30.3 ± 2ppm) versus the typical production testing range of 0 to 5 ppm was tested (See Figure 4). This was done so that the operator could observe the instruments upscale reaction during the weekly calibration schedule. This in-situ oxygen calibration process, which cannot be done with a dew point instrument, has already identified several failing dew-point cells.
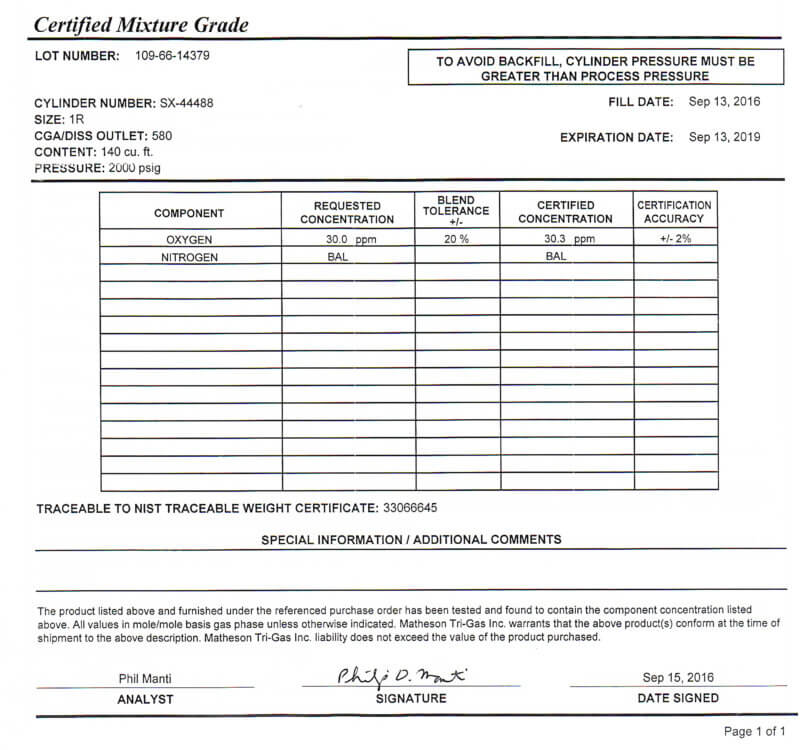
Figure 4
Oxygen and Water in Vacuum Systems
It is well known by any vacuum practitioner that the primary residual gas that is prevalent during a pump down of a vacuum furnace is dominated by water vapor. The water molecules from the atmosphere, bombards and clings to the inner surfaces of a vacuum system anytime a vacuum furnace door is opened. The longer the vacuum door is left open the more saturation will occur. Besides being detrimental to materials being heat treated, water vapor desorption or pumping time can be a major time-wasting problem in vacuum processing.
Once the vacuum furnace is completely pumped down and water vapor is minimized, the relative leak tightness of the vacuum furnace needs to be addressed. A vacuum furnace with a high leak rate will always possess higher residual oxygen content. Therefore, depending upon the size of the furnace, leak rates should never exceed 10 microns per hour at a pressure of 70 microns or less. A leak tight system, one where the volume of oxygen leaking into the furnace is sufficiently low, is paramount for consistent and accurate heat treatments of any materials.
Oxygen Versus Water – What is More Forgiving
Thankfully, oxygen and water vapor is always found in the air around us. However, the amount of water vapor will vary with temperature and atmospheric pressure. The earth’s atmosphere has the maximum capacity to hold a maximum volume of 2% water, regardless of temperature and pressure. In contrast, the earth’s oxygen content is 10 times that of water content (see Table 1). Additionally, the vacuum processing of critical components can only tolerate up to 2.5ppm of oxygen, compared to 10ppm of water vapor. Therefore, water vapor is much more forgiving in vacuum processing than oxygen.
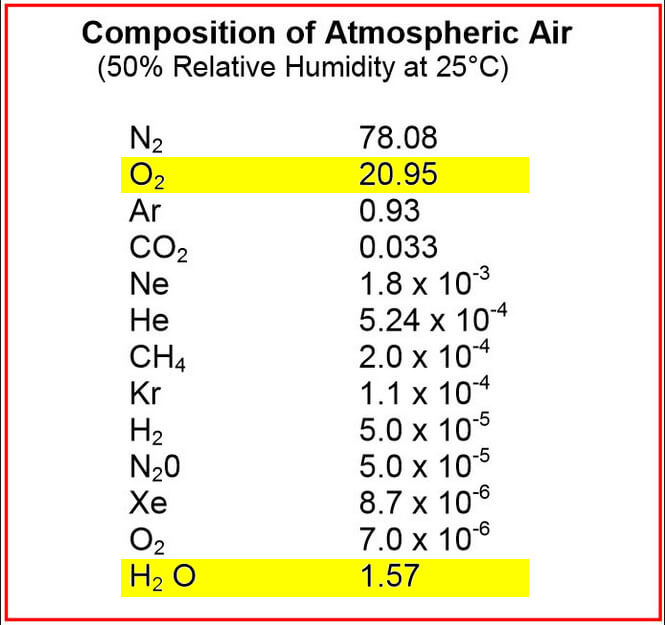
Table 1
Conclusion
It is indisputable that water vapor (dew point) and oxygen are both problematic actors in vacuum processing. Currently all technical specifications are only addressing one of these measurements in our specialty gas usage, dew point. Given the natural inconsistencies of dew point readings, the lack of in process calibration of dew point instrumentation, and the abundance of oxygen in our atmosphere – are we really analyzing the correct elements?
For specification requirements, this author continues to record daily dew point readings for every specialty gas. However, for true verification of critical clean and dry inert gases being used in vacuum processing, this author exclusively assesses the oxygen content results derived from the Oxygen Trace Analyzer instrument.
Written by
Bob Hill, President of Solar Atmospheres of Western PA and Solar Atmospheres of Michigan