Dew Point Versus Oxygen Content In Vacuum Processing
Dew Point Versus Oxygen Content In Vacuum Processing Introduction
It is well known that accurate measurement of any heat treating atmosphere can have a significant effect on the quality and process yield of heat treated components. Traditionally, dew point analysis has always been the bellwether in determining our heat treating atmospheric conditions. This is because it was discovered very early on that moisture parameters can have a tremendous impact on carbon potential and thus on final properties. Since the main objective of any neutral furnace atmosphere is to prevent detrimental effects such as carburization, de-carburization, hydrogen embrittlement, oxidation, and soot formation, one must analyze much more than moisture content of a furnace atmosphere. Contending with such constituents as CO, CO2, H2, H2O, N2 and hydrocarbons (e.g. CH4), a better more robust instrument was needed to analyze endothermic or exothermic atmospheres. Today, either Oxygen Probes or Three Gas Analyzers are the industry’s preferred analytical instruments to determine carbon potential within an atmospheric furnace.
Problems with Dew Point Content In Vacuum Processing
As the analysis and controls of atmospheric furnaces have evolved over the years, this author has questioned the antiquated method of measurement of purity of specialty gases within the vacuum processing arena. Why is dew point measurement still the bellwether of specialty gas providers, and more importantly within the vacuum heat treating industry? With the advent of such innovative manufacturing processes as Additive Manufactured components, the new metallurgy that comes with AM parts requires ultra-clean atmospheres. (See Picture 1) Is the dew point analyzer really the best instrument that we have in our toolbox?

Analyzing Dew Point in Vacuum Heat Treat Furnaces
Examine this picture of a rose (see Picture 2). The appearance of liquid water on this rose occurs only because of one simple fact: the temperature on the surfaces of the rose petals collecting the dew is below the dew point of the surrounding air. Likewise, the amount of water within specialty gas sampling lines in terms of the number of molecules or mass of water determines at what temperature the water vapor starts to go into liquid phase.

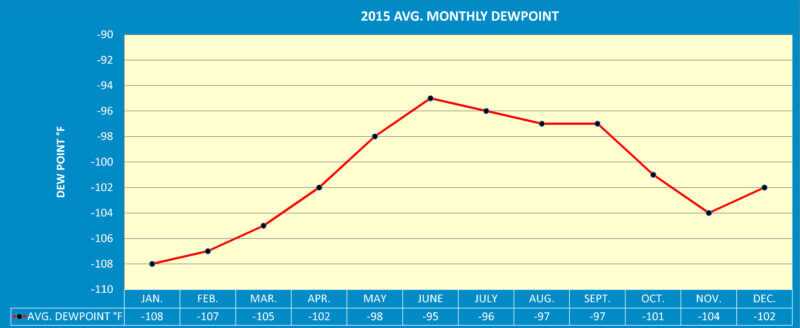
Therefore, an “exact” dew point measurement is most dependent upon the surrounding temperature. Note how the dew point values within the environs of a state of the art heat treatment plant process gas lines, i.e. N2, AR, etc., vary from summer to winter (see Graph 1). The fact is today’s heat treatment plants are probably the worst environment to test for dew point. The majority of heat treating facilities in the world experience tremendous swings of ambient temperatures and relative humidity even within a 24 hour period of time. (See Solar Atmospheres’ Publication No. 3 in its Vacuum Furnace Reference Series entitled “Operating a Vacuum Furnace Under Humid Conditions,”).
Trace Oxygen Analyzers
A trace oxygen analyzer is a versatile microprocessor-based instrument used for detecting parts per million (ppm) levels of oxygen. Oxygen sensing instruments are typically sealed units and require reasonably regulated sample pressures (.2 to 2.4 slpm). The response time is dependent upon the flow rate, e.g. a low flow rate will result in a slower response to O2. More importantly a trace oxygen analyzer results in a signal that is independent of temperature.

Solar Atmospheres engineers realized the advantages of trace oxygen instruments versus dew point instruments and decided to build a combination instrument that would employ both methods (See Picture 3). Solenoid controls automatically sample each of the four specialty gases (nitrogen, argon, helium and hydrogen) utilizing both instruments every six hours 24 hours a day. All dew point and trace oxygen results are recordable and traceable along with the plant’s ambient temperature and relative humidity. Alarm features are set for any values of dew point above -60° F and /or values of oxygen greater than 5 ppm in the process gas feed lines.
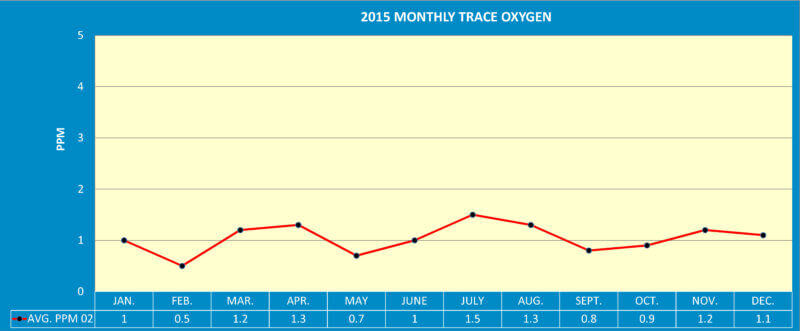
After one full year of side by side operation it was very clear to see the trace oxygen analyzer is the instrument of consistency (See Graph 2).
Specifications for Dew Point Measurements
So why has the vacuum heat treating community, when it comes to determining purity of their specialty inert gases, been slow to react when compared to the atmospheric heat treating community? It is this author’s opinion we are all being driven blindly by the specifications that govern us. Many specifications require only dew point measurements for gas purity.
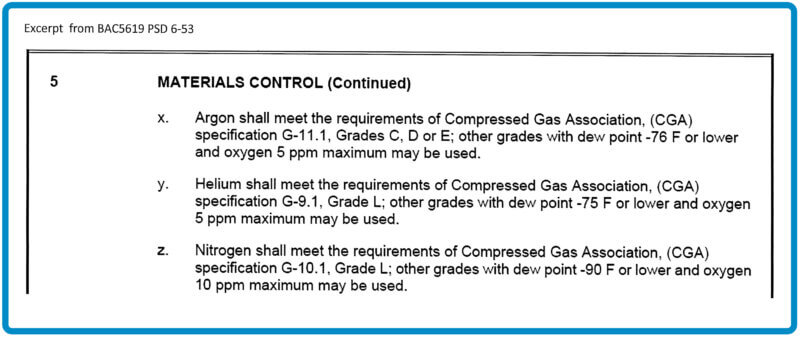
More recently some specifications, such as Boeing Aircraft, acknowledge the Compressed Gas Association’s designations which include dew point AND ppm of oxygen for various gases (See Table 1). However, AMS 2769B paragraph 3.2.1.1 counteracts this allowance by addressing only dew point issues, more specifically the installation of sampling lines, the location of the dew point cell, and recording frequencies (See Table 2). This paragraph is often fertile ground for “findings” by any auditors who perform outside accreditation audits on heat treating facilities.

Dew Point Versus Oxygen Content In Vacuum Processing Conclusions
When vacuum heat treating metal alloys that oxidize readily in the presence of small concentrations of water vapor or oxygen, data suggests that dew point should not be the stand-alone gas purity analyzer. Dew point only measures the water vapor, not oxygen in the gas line. Adding an oxygen analyzer as an additional quality tool provides the heat treat shop greater assurance that the process gas entering the furnace is of the highest purity and meets the specifications of the customer.
Written by:

